Introduction
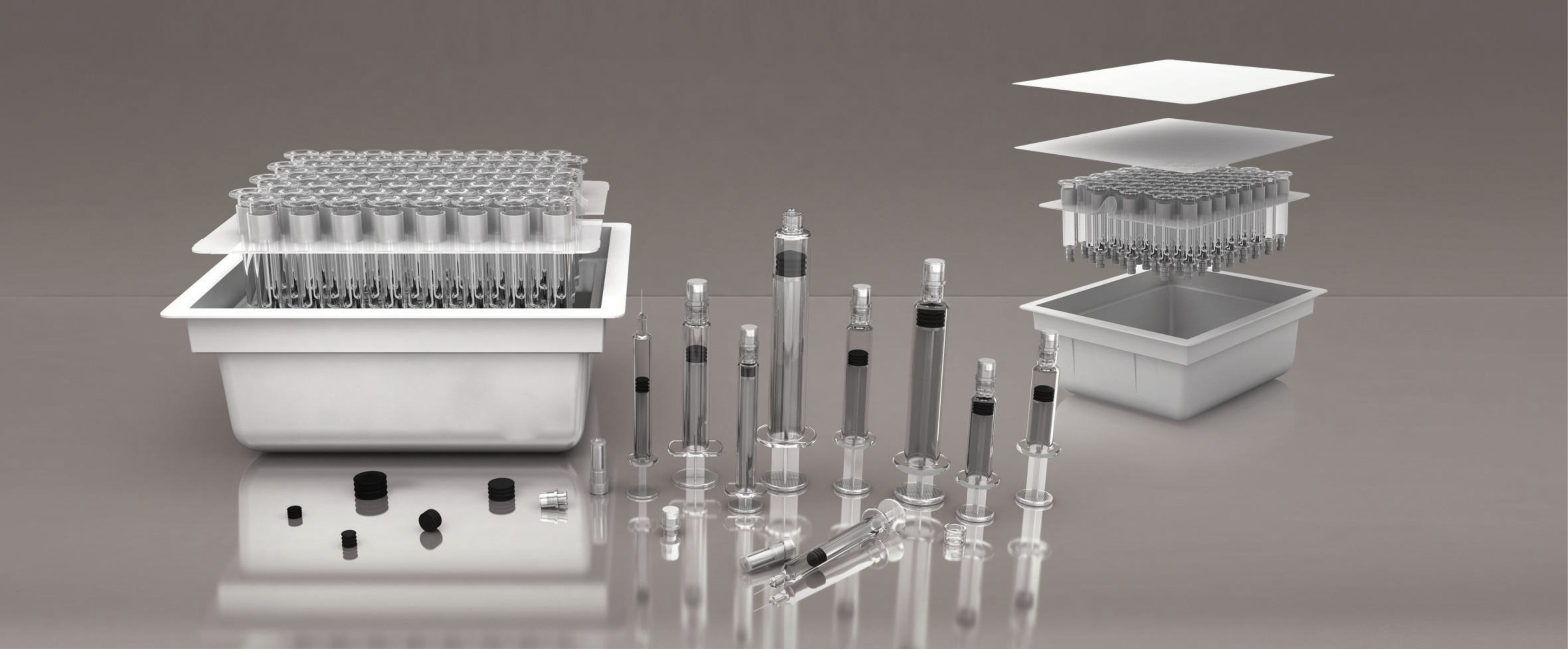
Vim Meditech Pvt. Ltd. pioneers in innovative parenteral drug delivery systems and it is the world's first company to have achieved manufacturing glass syringes and filling formulations into glass and polymer syringes from 0.5 ml to 10 ml - all under one roof.
Vim Meditech is one of the few companies in the world to manufacture glass Pre-filled Syringes, Pre-fillable Syringes, formulations and cytotoxic API, for captive consumption. Vim Meditech prides itself in its state-of-art manufacturing facility that meets the specifications of WHO-cGMP and other international regulatory bodies.
MISSION
To provide specialized pharmaceutical products PREFILLED SYRINGES which meet the highest quality, eligibility, safety and accessibility standards; to be environmentally-friendly and committed with the corporate social responsibility.
VISION
To reach an outstanding short-term leadership in the most important pharmaceutical specialties PREFILLED SYRINGES and, in a medium-term basis, to be the selection of choice for PREFILLED SYRINGES. In a long-term basis, we are determined to be acknowledged as one of the top solutions throughout the WORLD, for PREFILLED SYRINGES.
The benefits are numerous:

No chance of contamination from hand or from hospital atmosphere
Saves valuable time in critical care management
Easy to administer
Accurate dosage
Eliminates human errors of wrong choice of medication in an unmarked syringe
Minimal chance of needle injury to the healthcare staff
Minimizing medical wastage to a great deal by rendering vials and ampoules unnecessary and hence their production, avoidable.
RESEARCH & DEVELOPMENT
Ultra Modern Research Center
State-of-the-art research centre that has facilities to convert conventional molecules present in Vials/Ampoules into pre-filled syringes
Ultra-Modern Research Centre testing biocompatibility for over 272 drugs for PFS so far
Rigorous QA & QC department
More than 35 scientists and researchers are deployed for intense R & D
Product development is taken under structured timeline and associated studies are carried out simultaneously
INTERNATIONAL REGULATORY AFFAIRS
Dynamic & Futuristic Growth in Export Market
Regulatory Team:
An experienced and efficient International Regulatory Affairs (IRA) team helming the regulatory department
US-DMF is prepared and filed for empty PFS
Empty PFS registration and filing of documents for various countries is under process
Preparation and submission of dossiers is carried out for Export related Finished Products
Currently Dossiers are ready in CTD and ACTD format
Depending upon the country specific requirements dossiers are prepared
